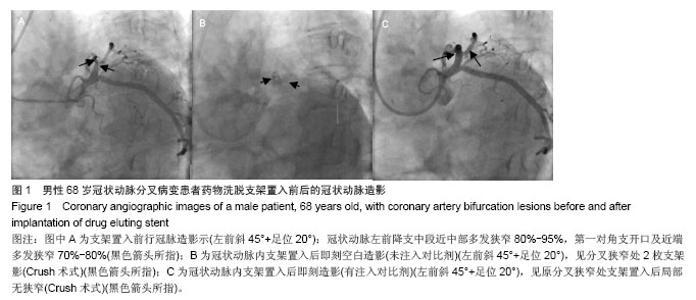
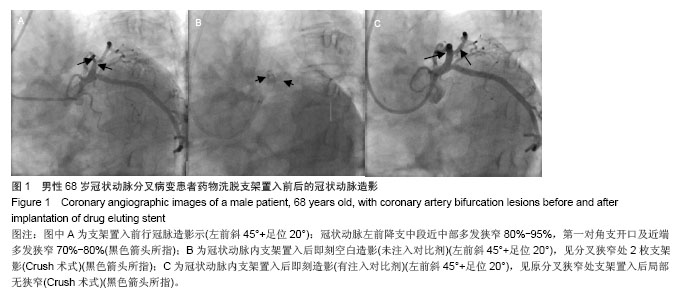
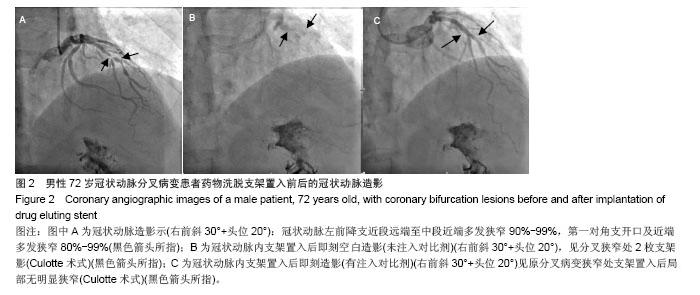
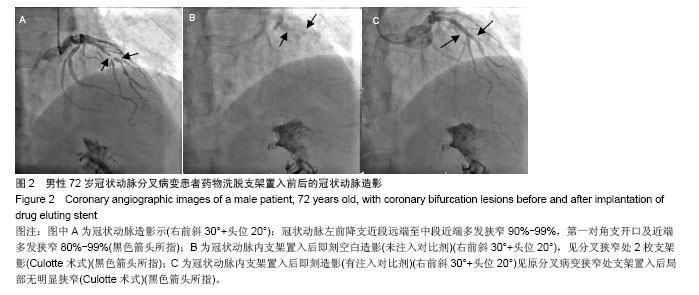
| [1]Latib A, Colombo A. Bifurcation disease: what do we know, what should we do? JACC Cardiovasc Interv.2008;1: 218-226.
[2]Daemen J,Wenaweser P,Tsuchida K,et al.Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369: 667-678.
[3]Tempelhof MW,Benzuly KH,Fintel D,et al.Eptifibatide-induced thrombocytopenia: with thrombosis and disseminated intravascular coagulation immediately after left main coronary artery percutaneous coronary angioplasty.Tex Heart Inst J. 2012;39(1):86-91.
[4]Marcucci R,Gori AM,Paniccia R,et al.Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119:237-242.
[5]Nguyen TA,DiodatiJG,Pharand C.Resistance to clopidogrel:a review of the evidence. J Am Coll Cardiol. 2005;45:1157-1164.
[6]Wu H,Qian J,Sun A,et al.Association of CYP2C19 genotype with periprocedural myocardial infarction after uneventful stent implantation in Chinese patients receiving clopidogrel pretreatment.Circ J.2012;76(12):2773-2778.
[7]陈绍良,高润霖.冠状动脉分叉病变介入治疗技术[M].北京:人民卫生出版社,2009:51-71.
[8]马长生.冠心病介入治疗-技术与策略[M].北京:人民卫生出版社,2005:584-585.
[9]中华医学会心血管病学分会,中华心血管病杂志编辑委员会.抗血小板治疗中国专家共识[J].中华心血管病杂志,2013,41(3): 183-194.
[10]Mehran R,Rao SV,Bhatt DL,et al.Standardized bleeding definitions for cardiovascular clinical trials:a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736-2747.
[11]Palmerini T,DangasG,Mehran R,et al.Predictors and implications of stent thrombosis in non-ST-segment elevation acute coronary syndromes: the ACUITY Trial. Circulation. Cardiovasc Int.2011;4:577-584.
[12]Bonello L,Tantry US,Marcucci R,et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010; 56:919-933.
[13]Zanger UM,Klein K,Thomas M,et al.Genetics, epigenetics, and regulation of drug-metabolizing cytochrome p450 enzymes.Clin Pharmacol Ther.2014;95(3):258-261.
[14]Kazi DS,Garber AM,Shah RU,et al.Cost-effectiveness of genotype-guided and dual antiplatelet therapies in acute coronary syndrome.Ann Intern Med. 2014;160(4):221-232.
[15]Arima Y,Hokimoto S,Akasaka T,et al.Comparison of the effect of CYP2C19 polymorphism on clinical outcome between acute coronary syndrome and stable angina.J Cardiol. 2014. pii:S0914-5087(14)00221-4.
[16]Barragan P,Bouvier JL.Resistance to thienopyridines:clinical detection of coronary stent thyrombosis by monittoring of vasosdilator-stimulated phosphoproteinphosphorylation. Catheri Cardiovasc Int.2003;59:295-302.
[17]WiviottSD,Antman EM.Clopidogrelresistance: a new chapter in a fast-moving story. Circulation.2004;109:3064-3067.
[18]Hokimoto S, Mizobe M,Akasaka T,et al.Impact of CYP2C19 polymorphism and proton pump inhibitors on platelet reactivity to clopidogrel and clinical outcomes following stent implantation.Thromb Res.2014;133(4):599-605.
[19]Bergmeijer TO,Janssen PW,Schipper JC,et al.CYP2C19 genotype-guided antiplatelet therapy in ST-segment elevation myocardial infarction patients-Rationale and design of the Patient Outcome after primary PCI (POPular) Genetics study. Am Heart J. 2014;168(1):16-22.
[20]Kara?niewicz-?ada M,Danielak D,Rubi? B,et al.The influence of genetic polymorphism of Cyp2c19 isoenzyme on the pharmacokinetics of clopidogrel and its metabolites in patients with cardiovascular diseases.J Clin Pharmacol.2014; 54(8): 874-880.
[21]Pare G,Mehta SR,Yusuf S,et al.Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Eng J Med. 2010;3 63:1704-1714.
[22]Wallentin L,James S,Storey RF,et al.Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010; 376:1320-1328.
[23]Ma TK,Lam YY,Tan VP,et al.Impact of genetic and acquired alteration in cytochrome P450 system on pharmacologic and clinical response to clopidogrel.Pharmacol Ther. 2010; 125(2): 249-259.
[24]Anderson JL,Adams CD,Antman EM,et al.2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e179-347. |